How Many Orbitals Are There in the 4p Subshell
The g subshell is outlined as having l 4 so m-sub-l could be any integer from -4 to 4. Total Number of Electrons in Shell.

There Are 3 Orbitals In A P Subshell Ml 1 0 Or 1 Ppt Download
How many orbitals are there in a 4p subshell.

. How many orbitals are 5f. How many orbitals are there in 4p subshell. Thus the s subshell has only one orbital the p subshell has three orbitals and so on.
2 6 10 18. Always remember that the magnetic quantum determines the number of orbitals in the subshells and generally s-orbital contains one orbital only as the value of azimuthal quantum number is zero p-orbital contains three orbitals d-orbital contains five orbitals and f-orbital contains seven orbitals. How many orbitals are there in the 4p subshell quizlet.
The f subshell has 7 orbitals. Remembering that the s subshell as 1 orbital and can hold 2 electrons the p subshell has 3 orbitals and can hold 6 electrons the d subshell has 5 orbitals and can hold up to 10 electrons and the f subshell has 7 orbitals can hold up to 14 electrons. What element is 3s2 3p1.
The p subshell has 3 orbitals. There is one s orbital and there are three p orbitals five d orbitals and seven f orbitals. Therefore the 4p orbital can hold two electrons and the 4p.
Energy shift orientation of the subshells shape Spin quantum number. Subshell s orbital is listed as 0 p orbital as 1 etc Magnetic quantum number projection of angular momentum mℓ. 3 An atom as a whole is electrically neutral because all charged subatomic particles reside in asked Jun 25 2017 in Chemistry by littleone.
2 6 8. The 4p subshell has 3. There are 2l1 orbitals in each subshell.
So for part a in a 4p subshell there are 3 orbitals present as the p subshell can hold up to 6 electrons. Spin of the electron 12 spin down 12 spin up. So it stands to motive that the g subshell has 9 orbitals.
Therefore the 4p orbital can hold two electrons and the 4p subshell can hold a total of six electrons. 9 OD 10 E. The 4p subshell contains 4px 4py and 4pz orbitals.
Maximum 6 electrons in 3 orbitals. 2 6 10 14 32 How many electrons can a 3d orbital hold. Now the 4p subshell contains a total of three 4p orbitals 4px 4py and 4pz.
Each orbital can hold two electrons so the. Do not confuse the number of orbitals in a subshell with the number of electrons the subshell can hold. Therefore you can say that a 4p orbital can hold a maximum of two electrons and the 4p subshell can hold a maximum of six electrons.
Therefore we can say that there are a total of three orbitals in 4p subshell. Explanation with p subshell l 1 ml -1 0 1 so there are three possible values of ml Remember that 4 is the number of principal quantum number it has nothing to do with the number of the orbitals. For your answer please just type the number.
There are 32 electrons in the 4th energy shell so there must be 16 orbitals in the 4th shell as the number of orbitals is half the number of electrons. The px py and pz each of which needs a maximum of two electrons of opposite spins as per the Aufbau Hunds and Pauli exclusion principles. 18 QUESTIONS What is the electron configuration of fluorine.
Question 12 1 pts How many orbitals are there in the 4p subshell. The d subshell has 5 orbitals. Critical Thinking Representation is a critical concept in democratic thinking.
Chemistry questions and answers. How many orbitals are there in the 4p subshell. As each orbital can hold maximum two electrons so total electrons p orbital can hold is 6.
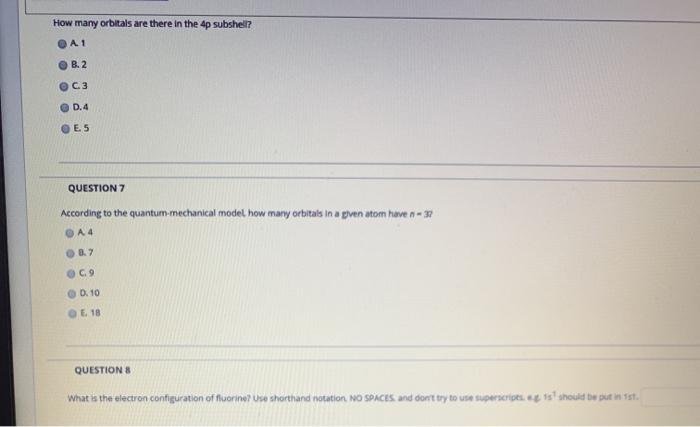
The 4p subshell contains 4px 4py and 4pz orbitals. 2 C3 D4 E5 QUESTION 7 According to the quantum mechanical model how many orbitals in a pven atom have n-3. How many orbitals are there in 4p sublevel.
How many orbitals are in the p subshell. For any atom there are seven 5f orbitals. Take a look at the illustration.
As each orbital can hold maximum two electrons so total electrons p orbital can hold is 6. 4s 4p 4d 4f. It does not matter if your energy level that is the coefficientnumber before the spdf orbital goes as high as 7 which is by far the maximum the number of suborbitals in p is always three.
See also how long can a walrus stay under water. Therefore the 4p orbital can hold two electrons and the 4p subshell can hold a total of six electrons. 2 The number of orbitals present in an electron subshell is always six.
View the full answer. What are the implications for a political system if it is left unclear whether elected legislators are responsible for functioning as. 3p orbitals 2 e 1p orbital 6 e.
Three possible orientations - There are five possible orbitals in a d subshell and 7 possible orbitals in an f subshell. If you type anything other than the number your answer will be counted as incorrect. This number divides the subshell into individual orbitals which hold the electrons.
The f-orbitals are unusual in that there are two sets of orbitals in common use. Thus the answer is B. Now the 4p subshell contains a total of three 4p orbitals 4px 4py and 4pz.
Use shorthand notation NO. In the hydrogen atom which of the following orbitals has the lowest energy. Since each of those p orbitals can hold a maximum of two electrons the p subshell can hold a maximum of.
There are 2l1 such values of ml for a given l and thus there are 2l1 orbitals in each subshell. How many electrons are in 4p orbitals. What are the N and L quantum numbers for the 4p subshell.
Which of the following subshell contains only one orbital.
Solved How Many Orbitals Are There In The 4p Subshell A1 B Chegg Com


0 Response to "How Many Orbitals Are There in the 4p Subshell"
Post a Comment